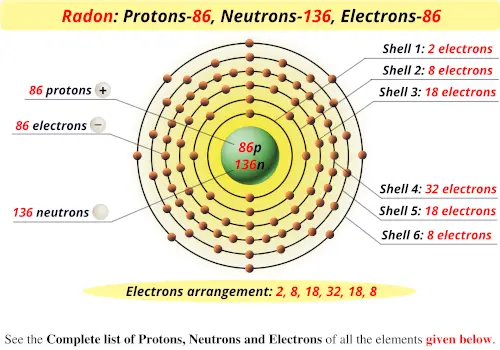
How Many Protons and Electrons Are in Radon
The number of neutrons is different for each isotope. The chemical symbol for Radon is Rn.

Radon Protons Neutrons Electrons Electron Configuration
How many protons and electrons are in radon.

. Radon has 86 protons 136 neutrons and 86 electrons. Radon has 86 protons and electrons. Now we will calculate the number of neutrons in the radon isotope.
973 gcm 3 Color. The atomic number 88 is the number of protons. Radon gas is considered a health hazard as it can accumulate in basements and radioactively decay to expose inhabitants to radiation which has been shown to cause lung cancer.
No matter how many electrons or neutrons an atom has the element is defined by its number of protons. Which element is it. How many protons and electrons are in each atom.
Astatine has 85 protons 125 neutrons and 85 electrons. In fact its actually possible to have an atom consisting of only a proton ionized hydrogen. The total number of neutrons in the nucleus of an atom is called the neutron number.
How many protons and electrons are in an atom of radon. 86 electrons white successively occupy available electron shells rings. 86 electrons white successively occupy available electron shells rings.
Radon has 86 protons and electrons in its structure. This is because isotopes have same no of electrons. Protons neutrons and electrons of all elements are mentioned in the table below You will get the List Shell diagram of all the elements.
The atomic number of Radon is which means that it has 86 protons in the nucleus since the number of protons is equal to the number of electrons in the neutral state so the total number of electrons is also 86The total number of neutrons in the nucleus is 136. How many protons neutrons and electrons does Po-218 possess. Watch More Solved Questions in Chapter 4 Problem 1 Problem 2 Problem 3 Problem 4 Problem 5 Problem 6 Problem 7 Problem 8 Problem 9 Problem 10 Problem 11 Problem 12 Problem 13 Problem 14 Problem 15 Problem 16.
Xe 4f14 5d10 6s2 6p6 What compounds is radon found in. See also What does orale mean. Does radon have electrons and protons.
The proton number and electron number are the same which is 86 View Answer Discussion You must be signed in to discuss. Particle 2 protons and 2 neutrons. Protons 86 neutrons 222 - 86 136 electrons 86 Long Answer Questions Q9 Why do isotopes show similar chemical properties.
Radon is a noble gas in group 18 period 6 and the p-block of the periodic table. Table of Content How many protons and neutrons electrons does silicon have. Up to 24 cash back How many protons neutrons and electrons are there in one atom of this radon isotope.
Is radon denser than air. Neutrons are completely separate from. Noble Gas Crystal Structure.
Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The periodic table is arranged in order of increasing atomic number so the number of protons is the element number. The nucleus consists of 86 protons red and 136 neutrons orange.
One of the decay biproducts is Polonium-218. Radon is a noble gas and a nonmetal Protons. Radon has 86 protons and electrons.
Which element is it. How many neutrons do protons have. Number of neutrons Atomic Mass of the Rn isotope - 86.
How many protons P and neutrons N are in an atom of radium ra that has a mass number of 226. Radon is a chemical element with. The mass number 226 is the total number of protons and neutrons.
Copper has two isotopes copper-63 and copper-65. Radon - Protons - Neutrons - Electrons - Electron Configuration. 86 Neutrons in most abundant isotope.
Mass numbers of typical isotopes of Radon are 222. TextNo of neutrons text Atomic mass-no of protons No of neutrons 222 - 86 No of neutrons 136 Hence the no of Protons Neutrons and electrons in _86222Rn isotope are 86 136 and 86 respectively. Radon 86 protons and 86 electrons magnesium 12 protons and 12 electrons.
Do the atoms in the figure have the same atomic number. Radon is a noble gas in group 18 period 6 and the p-block of the periodic table. 13 rows Atomic Number Protons Electrons and Neutrons in Radon.
One of the decay biproducts is Polonium-218. The nucleus consists of 86 protons red and 136 neutrons orange. 4 222 nucleons and 222 86 136 neutrons.
What is proton and neutron. Cubic Density 293 K. 2220 amu Melting Point-710 C 20215 K -958 F Boiling Point-618 C 21135 K -7924 F Number of ProtonsElectrons.
An atom contains 66 electrons. In either case it simply designates the element radon which always has 86 protons and that the particu- lar isotope of radon is the one with 136 neutrons. An atom contains 14 protons.
Isotopes and Mass Number Isotope Mass number - Example 1. 86 Number of Neutrons.

Radon Atomic Structure Stock Image C018 3767 Science Photo Library

Radon Atomic Structure Stock Image C045 6433 Science Photo Library

Radon Atomic Structure Stock Image C023 2592 Science Photo Library

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Thorium Electrons

Radon Protons Neutrons Electrons Electron Configuration

Vektor Stok Vector Radon Atom Tanpa Royalti 498849847

Webelements Periodic Table Radon Properties Of Free Atoms

Radon Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered

Radon Measurement Operators Proficiency Course Ppt Video Online Download
Protons Neutrons Electrons Of All Elements List Images

Radon Protons Neutrons Electrons Electron Configuration

Rn Radon Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

222 Rn 86 Is An Isotope Of Noble Gases Radon How Many Protons Neutrons And Electrons Are There In Brainly In

3d Render Of Atom Structure Of Radon Isolated Over White Background Protons Are Represented As Red Spheres Neutron As Yellow Spheres Electrons As Blue Spheres Stock Photo Picture And Royalty Free Image

222 Rn Is An Isotope Of Noble Gas Radon How Many Protons Neutrons And Electrons Are There86in One Atom Of This Radon Isotope Snapsolve

3d Render Of Atom Structure Of Radon Isolated Over White Background Protons Are Represented As Red Spheres Neutron As Yellow Spheres Electrons As Bl Stock Photo Alamy
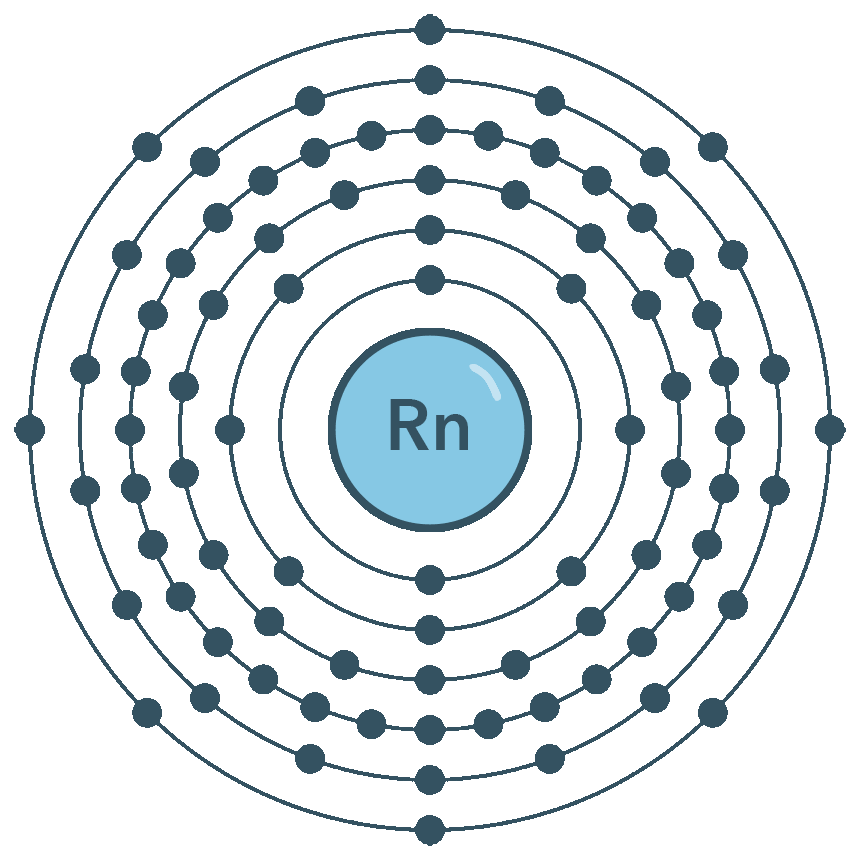
Learn About Radon Gas Air For Kids

Radon Atomic Structure Stock Image C013 1643 Science Photo Library

Comments
Post a Comment